- Contact Person : Ms. Ding Helen
- Company Name : Hangzhou Singclean Medical Products Co., Ltd.
- Tel : 86-571-63431880,13989831828
- Fax : 86-571-63431886
- Address : Zhejiang,Hangzhou,Gaoqiao Industrial Park (Hefeng)
- Country/Region : China
- Zip : 311402
restylane cross polymer hyaluronate dermal filler for plastic surgery
1.singfiller strengths
1 No-animal cross-linked hyaluronic acid filler
2 unlike rooster-derive hyaluronic acids and bovine collagen items,singfiller is free from animal proteins ,this effectively avoids any risk of animal -based disease tranmissious or allergic reactions to animal proteins
3 BD syringe and needle or your package demands
4 take fermentation technology to produce hyaluronate acid powder
2.Medicinal mechanism
singfiller cross-liked hyaluronic acid is water loving as it gradually degraddes ,each molecule binds to more water and over time. this character helps to a long-lasting results
3.application:
removing facial wrinkles
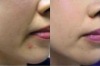
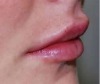
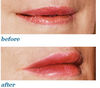
enhancing lips
humpnose
lung chin
lip implants
abundant temple
abundant mandible
abundant cheek
abundant vermilion tubercle
shaping facial contours
Breast augmentation
4.packing
1ml/syringe,2ml/syringe,4ml/vial,10ml/vial,100ml/vial,500ml/vial,1000ml/vial
5.specification:
| Item | size of particles(mm) | concentration | needle | syringe(BD) |
| Perfectha Derm Finelines | 0.1~0.15 | 20mg/ml | 30G | as requested |
| Perfectha Derm | 0.15~0.28 | 20mg/ml | 27G | as requested |
| Perfectha Derm Deep | 0.28~0.5 | 20mg/ml | 27G | as requested |
| Perfectha Derm Deep Plus | 0.5~1.25 | 20mg/ml | 26G | as requested |
| Perfectha Derm Sub-Skin | 1.25~2.0 | 20mg/ml |
6.welcom to visit us :
7. certification:
restylane cross polymer hyaluronate dermal filler for plastic surgery